-
NewsNews
-
Filter technology
Filter technology
-
Hose systems
Hose systems
-
Publications
Publications
-
Trade fairs
Trade fairs
-
Filter technology
-
Textile filtrationTextile filtration
-
Filter cloths
-
Filter bags
Filter bags
-
Filter belts
Filter belts
-
More products
More products
-
Service filter technology
Service filter technology
-
Areas of application filter technology
Areas of application filter technology
-
Filter cloths
-
Hoses technologyHoses technology
-
Hoses
-
Couplings & connections
Couplings & connections
-
Accessories
Accessories
-
Service hoses technology
Service hoses technology
-
Areas of application hoses technology
Areas of application hoses technology
-
White Paper
White Paper
-
Hoses
-
Other applications
Other applications
-
About usAbout us
-
Markert Group
Markert Group
-
Kunstforum Markert
Kunstforum Markert
-
Management & advisory board
Management & advisory board
-
Research and development
Research and development
-
Quality and the environment
Quality and the environment
-
Production system
Production system
-
Markert Group
-
CareersCareers
-
Training
Training
-
Jobs
Jobs
-
Work at Markert
Work at Markert
-
Training
-
Contact
Contact
Requirements for PTFE hoses in the pharmaceutical, food and chemical industry
Innovation through years of research and development
Hoses are omnipresent in pharmaceutical and chemical manufacturing. By comparison with a garden hose from a DIY store, with industrial hoses there are complex questions to answer and approval processes to go through. These are comparable to those for mechanical systems and machines. What needs to be considered for industrial hoses? What is considered as state of the art?
Hoses, like all the production equipment in a system, need to be designed for their application and have to be suitable for their intended use. Furthermore, they must not negatively impact the quality of the transported medium.
Just as material certificates are normally required for pressurized hose lines, there must be the correct documentation for hoses in contact with product or pure media. In addition, there is a whole series of relevant certificates. For pharmaceutical and food applications additional standards apply, such as USP Class VI, 3-A or FDA.
Before being taken into use, hoses must go through a qualification process. The following points are important in this context: Cleaning, disinfection, sterilization, intended use (single/multi-use), poss. leachout, inner surface test, particle release, durability and passing test procedures.
1. PTFE hose design
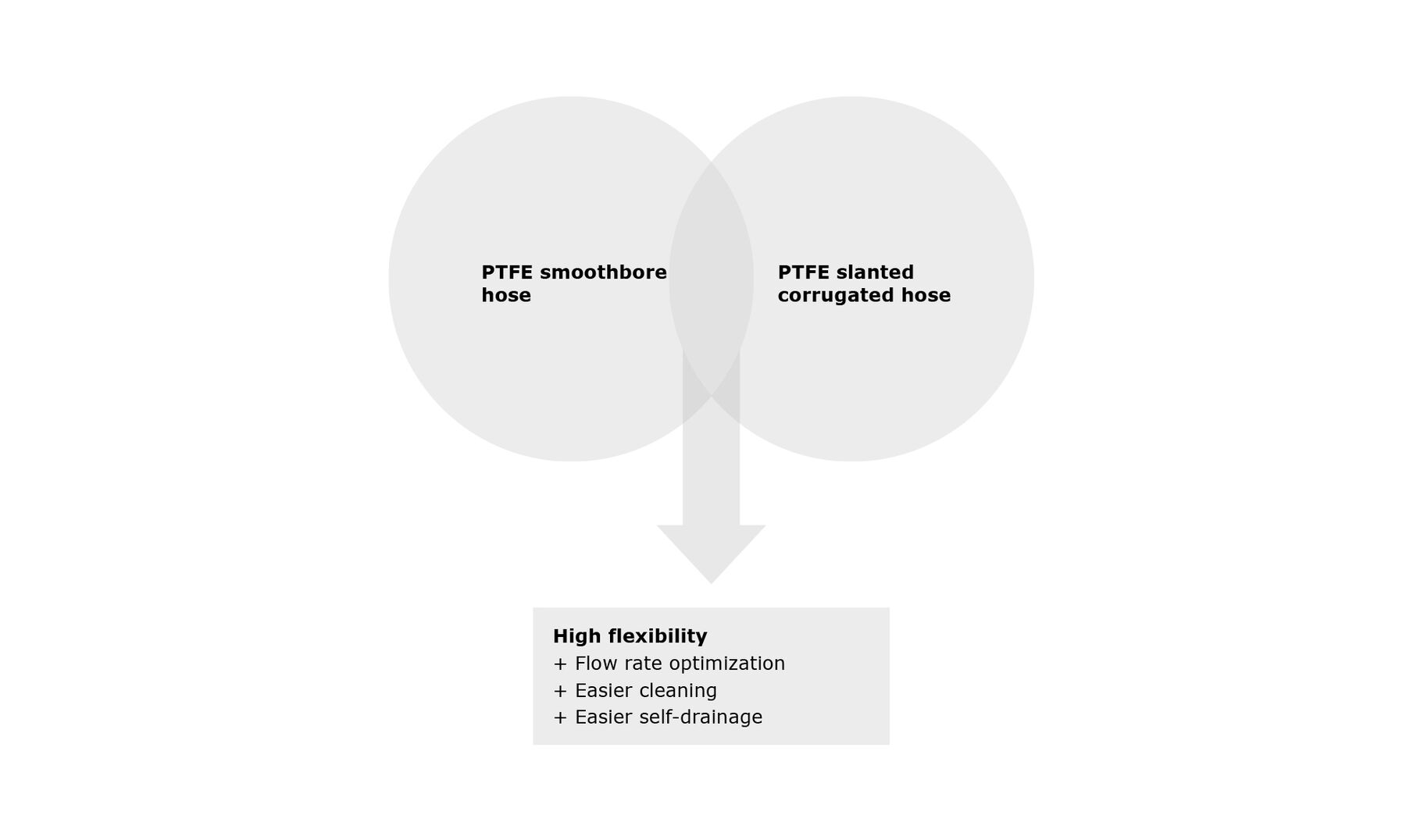
PTFE hoses have been in use in the pharmaceutical and chemical industries for over 30 years. A distinction is made between corrugated and smooth PTFE hoses. Each has its specific properties for different applications.
25 years ago these were combined in the form of PTFE hoses corrugated on the outside and smooth on the inside.
2. Vacuum resistance
The design of the HygienicPureFlex gives it the following advantages:
- Much lower penetration risk (since there is no stainless steel spiral directly adjacent to the outer wall of the PTFE liner)
- Substantial weight reduction
- No danger of liner damage from a broken spiral
And all of that with full vacuum resistance!
3. Robustness of the hose
To ensure long durability of a hose, it must be designed for the pressure, temperature and medium to which it will be subjected.
The special design challenge lies in getting the ideal combination of wall thickness, surface geometry and the desired bending radii. This is the only way to get the optimum robustness and handling.
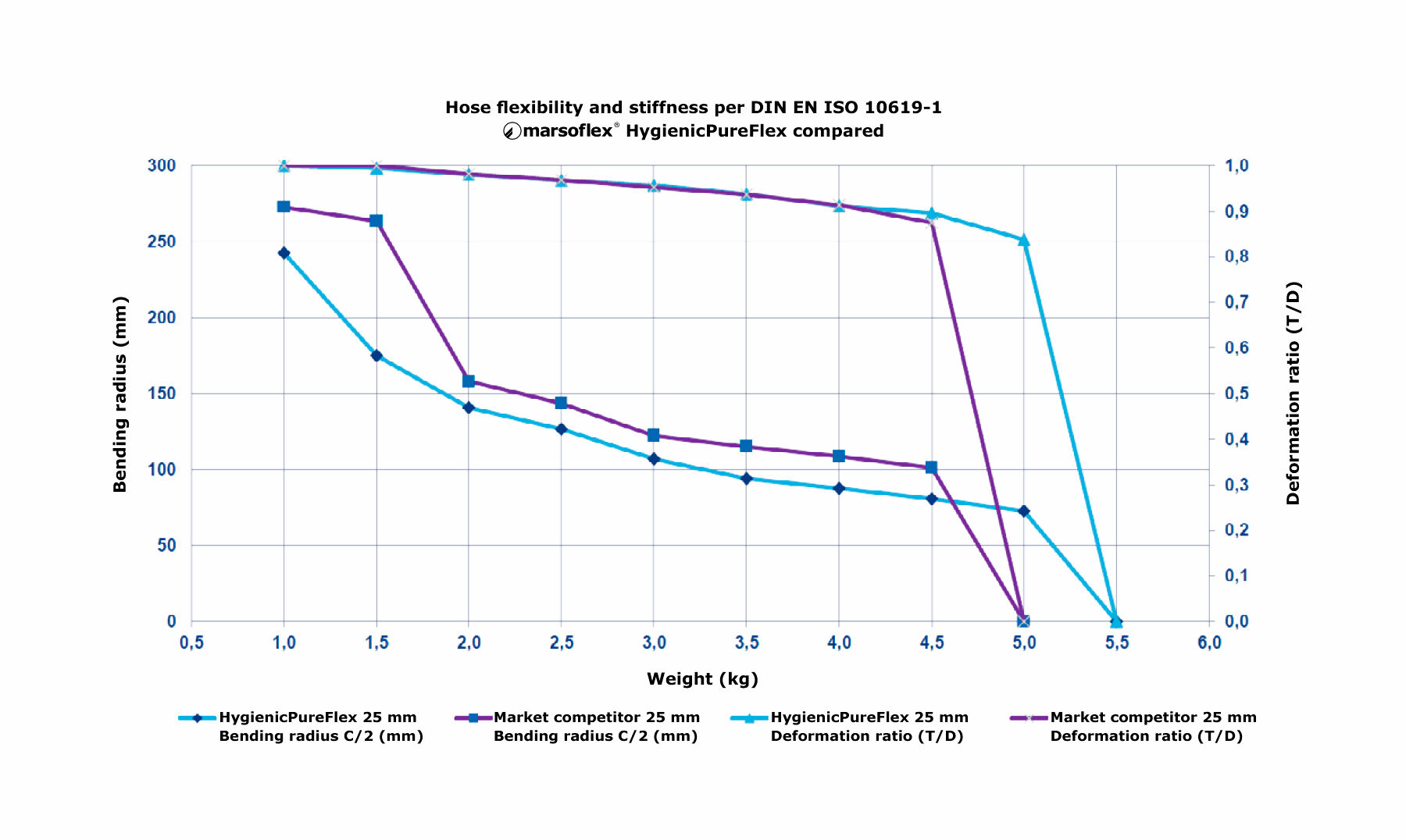
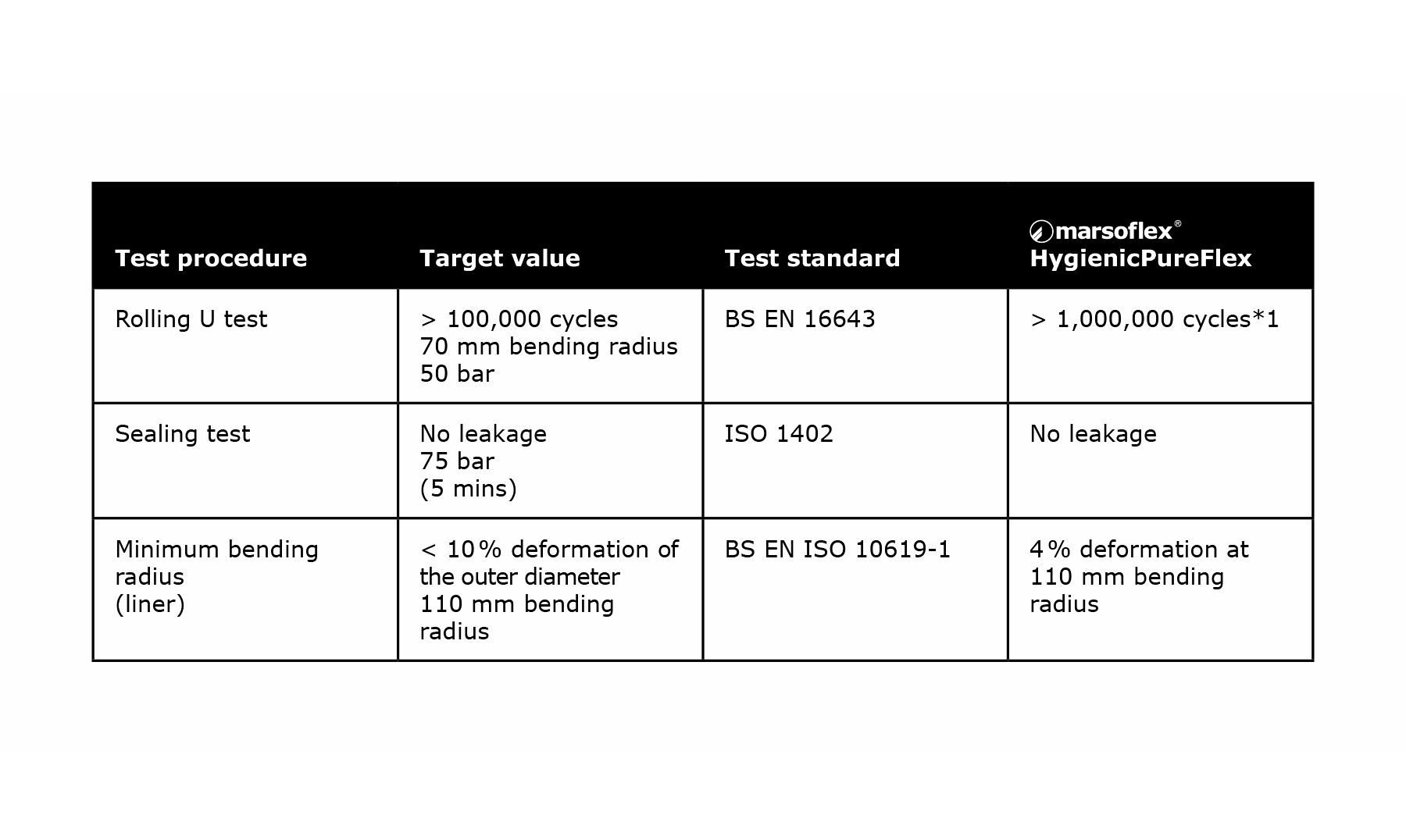
With HygienicPureFlex a standard 10 % higher robustness is assured, compared to other products available on the market. This has been confirmed in extensive testing.
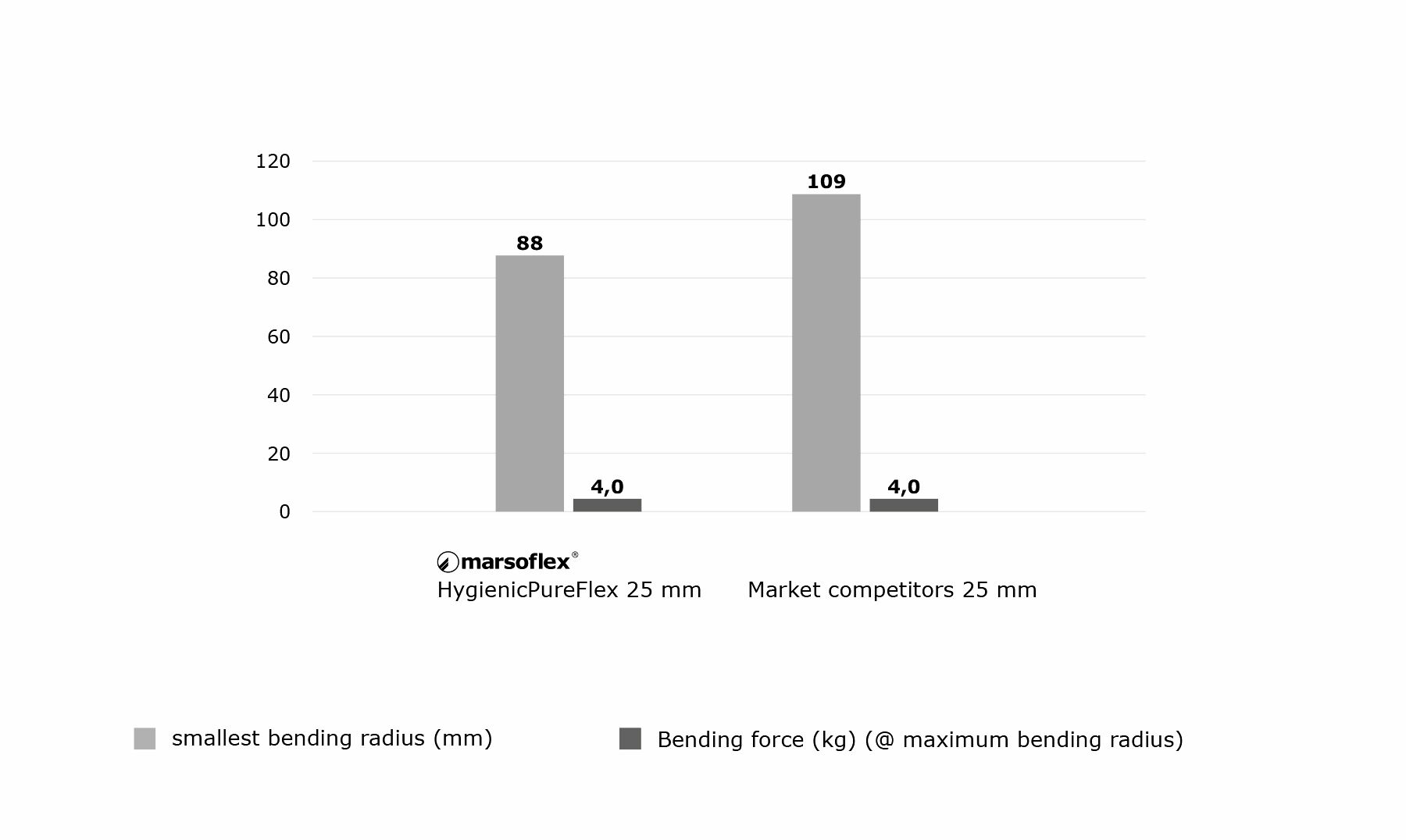
4. Flexibility of the hose
If hoses are not firmly installed they must be flexible in order to ensure good handling. This flexibility depends on the bending radius and on the force necessary to bend the hose.
The combination of smooth and corrugated PTFE hose is intended to achieve just this product characteristic. The surface geometry of the HygienicPureFlex hose is optimized for above-average flexibility. This has been technically demonstrated by appropriate bending tests.
6. Cover qualities
Stainless steel wire braid:
A stainless steel wire braid protects the PTFE liner while ensuring high pressure resistance of the hose. It improves the bending strength. Typically, high-quality stainless steels such as 1.4301 are used. The braid is abrasion- and scuff-resistant. The stainless steel wire braid can further be provided with a rubberized cover of CR or a silicone cover.
Polypropylene braid:
If 1.4301 is insufficiently resistant to an aggressive outer atmosphere, polypropylene braids can be used as pressure carriers for PTFE hoses. B6 polypropylene braid of specially developed monofilaments stands up to high operating pressures, while its low intrinsic weight provides for easy handling. It can be used in environments that are hostile to metals. Due to the electrical conductivity of the braid design, polypropylene braid can also be used in Ex Zone 0.
PESC braid:
For environments with not only aggressive atmospheres but also temperatures over 80°C, PESC braids are used. PESC is a polymer developed for uses requiring a high-performance fibre. It is highly dimensionally stable and chemical resistant, and can be used in temperatures up to 230°C. Stainless steel fibres (4-8 strands of 1.4404) provide electrical conductivity.This makes PESC braid usable in Ex Zone 0 as well.
7. Documentation and certification
Pressure test certificate:
Per the Pressure Equipment Directive (PED) all pressure equipment, including hoses, must be tested. The hose is subjected to 1.5 x the operating pressure by means of water or another medium. The data is recorded in the test certificate, which is a mandatory part of the product documentation.
―› The test certificate for hoses, chapter “Test Certificate per DIN EN 10204 3.1 – Pressure Test” confirms successful pressure testing.
Conductivity test:
Electrical conductivity is often an important property of hoses. The resistance of the hose is tested to determine its conductivity, and the results recorded in the test certificate.
―› The test certificate for hoses, chapter “Test Certificate per DIN EN 10204 3.1 – Conductivity” confirms that the results of conductivity testing were within the specified tolerances.
FDA conformity:
The US FDA (Food and Drug Administration) is the regulatory agency responsible for monitoring all goods brought onto the US market. This covers all imports, for which reason the directives and decisions of this agency are also important for manufacturers from other countries. They are binding for exports to the USA, since not every product approved for use in Germany or the EU is automatically FDA-compliant. Product manufactures therefore increasingly follow FDA standards, in order to compete in international business. FDA conformity requires materials with high durability that release no substances into foods. The food industry uses many plastics with very different characteristics, that come into contact with foods. Hoses are used in food manufacture primarily to transport liquids and pastes. Of most importance in this context is the chemical resistance of the material to substances in foods like fatty acids or fats. Furthermore, food industry hoses must have smooth interior surfaces, so that they can be cleaned readily and to prevent bacterial contamination.
The FDA regulations are divided into several sections or titles. FDA21 stands for Food and Drugs, CFR stands for Code of Federal Regulations and Part 177 for Indirect Food Additives: Polymers. This section of the FDA regulations describes the permissible polymers.
―› The certificate of conformity confirms that only FDA-approved polymers are used in the liner of the hose.
USP Class VI:
Plastics used in medical technology and pharmaceutics are divided into six biocompatibility classes in United States Pharmacopeia (USP), the US medications handbook. To assign elastomers and other polymer materials to a class, they are subjected to various tests in order to determine their biological reactivity in living organisms. The main goal of the USP is to maintain and improve human health. It is intended, by means of binding directives for the manufacture of medications and medical products, to ensure the quality of the substances inspected. This heightens the safety of patients and consumers. It evaluates the correct identity of the medication, active ingredient strength, quality, purity and composition. The standards are used by manufacturers as well as regulatory authorities. Prescription as well as non-prescription medications must meet USP-NF standards by US Federal law. In order to gain USP Class VI status, the material itself and its various extracts are subjected to the following tests in external laboratories.These tests can be divided into the following three test areas:
- Acute systemic toxicity: Determination of acute irritation from skin contact, inhalation and swallowing.
- Intracutaneous reactivity: The test material is brought into direct contact with the tissue for which its normal use is intended.
- Implantation test: The reaction after implantation in the tissue of a living organism is investigated. The duration is five days, as a rule.
Testing is done with defined exposure times and at defined temperatures, in order to ensure comparability of the results. Although biocompatibility testing must be done with the finished medical product, for the manufacturer it is important that all materials used to make the product are tested, and meet the requirements of the finished product.
USP Class VI is further subdivided in detail per EP 3.1.9. This subcategory calls for specific tests of silicone elastomers, such as the residual peroxide content.
―› USP Class VI certification confirms that the hose body material has successfully passed testing per USP Class VI.
European regulation 1935/2004:
The basic requirements for materials and objects intended for contact with foods (as defined by the LFGB1) are laid down in framework regulation (EU) No. 1935/2004. All food contact materials must meet these general requirements. Thus: “Materials and articles, including active and intelligent materials and articles, shall be manufactured in compliance with good manufacturing practice so that, under normal or foreseeable conditions of use, they do not transfer their constituents to food in quantities which could endanger human health; or bring about an unacceptable change in the composition of the food; or bring about a deterioration in the organoleptic characteristics thereof.” (Article 3 of regulation (EU) No. 1935/2004).
―› EU 1935/2004 confirms that the hose material meets the requirements of 1935/2004 for use with foods.
BfR XV. Silicone:
The EU directives are based, among other things, on the recommendations of the German Federal Institute for Risk Assessment (BfR). This results in further-reaching equirements for materials such as silicone (BfR XV.Silicone). Here, specific requirements for starting materials like silicones are set forth.
―› This requirement/rule applies only when silicone is used in the hose.
3-A Sanitary Standard:
3-A Sanitary Standards Inc. (3-A SSI) is an independent, non-profit organization in the US whose purpose is to improve hygiene design in the food, beverage and pharmaceutical industries. The 3-A symbol is administered by 3-A SSI and is a registered mark that identifies operating materials that meet 3-A Sanitary Standards. These standards are defined for the design, manufacture and cleanability of daily food equipment. They come into force when such equipment is used in the handling, production and packaging of consumable products with high hygienic requirements. These hygiene standards are the result of joint efforts by industry experts, production system manufacturers, food and beverage producers and hygiene regulatory authorities. The goal of 3-A SSI is to protect edible or drinkable consumer goods from contamination, enable mechanical cleaning of all product surfaces and simple disassembly for manual cleaning. 3-A standards are recognized and accepted worldwide.
―› The implementation of hygiene standards for food use is confirmed.
ISO 10993:
ISO 10993 describes the most important test procedures for the biological evaluation of the cytotoxicity of medical products. Cytotoxicity is the ability of a substance to damage or destroy a living cell.
―› ISO 10993 confirmation certifies performance of the test procedures described in the standard.
8. PTFE lining
To integrate hose lines into processes, normally fittings are used. The fitting can be attached to the hose by being pressed in or by lining. In lining, the PTFE hose is warmed to make it malleable. In this condition it is flared through the fitting or coupling and then folded (tafted) back over the end of the connection, i.e. fitted to the connection. The advantage in this process is that there is no dead space in the transition area from fitting to hose in which material could collect. This provides the highest possible cleanliness in sensitive applications in pharmaceutical and food uses.
Further benefits
- Entire sealing surface covered in PTFE
- Minimum dead space
- Antistick effect
- Corrosion resistance even against very aggressive substances like acids and bases that corrode stainless steel
- Almost unlimited hose life
Many different fittings can be lined:
- Loose flange/fixed lining
- Lever arm/camlok
- Dairy pipe
- Tank truck
- TriClamp
―› The function and durability of the lining can be further improved by using a PTFE protective cap / protector.
―› PTFE inserts can be used for fittings that cannot be directly lined (like step sizes).
Often, the entire raised sealing diameter cannot be covered by a hose flange. In order to ensure consistent high quality, Markert therefore uses an automated lining process that ensures complete sealing face coverage (called standard flange).
9. Cleaning and testing procedures for hoses in pharmaceutical applications
Hoses in pharmaceutical applications must always be cleaned and inspected before every use. The following procedures are commonly used in the industry:
TOC analysis (Total Organic Carbon)
There are various ways to determine the TOC (Total Organic Carbon). The one most frequently used is the direct or NPOC (Non Purgeable Organic Carbon) method. In it, first the inorganic carbon compounds are eliminated, such as carbonate, hydrogen carbonate and carbon dioxide. For this purpose a mineral acid like hydrochloric acid is added to the water sample. The carbonate and hydrogen carbonate are converted into carbon dioxide and water, and then all carbon dioxide is removed from the sample using a flushing gas. Determining the TOC in this way is simple and quick, and takes only a few minutes. Furthermore, there is no need for time-consuming sample preparation. In addition, this TOC analysis captures not just a single compound, but many compounds. This makes it product-independent and highly flexible, for products that contain organic compounds.
Foods are generally not pure substances, but are made up of many organic components, such as carbohydrates, fats and proteins. TOC analysis also captures the surfactants used for cleaning, in addition to the product itself.
In indirect sampling, after cleaning there is a final rinse with water. This final rinse water is then analysed. The advantage of the final rinse method is its speed, as the water is simply drawn into a container and analysed.
CIP (Cleaning in Place)
Cleaning in place is a procedure for cleaning production systems and hoses. The system is cleaned in a cycle or flushing process, without prior disassembly. The cleaning process differs according to the product being processed, for example foods, beer or dairy products. Typically it includes the following steps:
- Pre-flushing with water to remove coarse dirt
- Flushing with detergent or cleanser
- Flushing with water to remove the cleanser
- Flushing with acid • Final rinse with water
- Optional: Disinfection, for example by post-rinse disinfection in which the system is left filled with disinfectant after production, for example over a weekend.
SIP (Sterilization in Place)
Sterilization in place is the sterilization of production systems without prior disassembly. Normally sterilization is by means of hot steam. SIP systems are used in applications where bacteria reduction is critical. Such applications include biotechnology and the food and beverage industry. Sterilization is performed using steam at 120°C and 2 bar for a period of about 60 to 70 minutes. The duration depends on the application area and type of system.
Gamma ray sterilization
High-energy gamma radiation causes electrons to split off from atoms (ionization) in any object it hits. In living cells this ionization damages the DNA and other cellular structures. These changes at the molecular level caused by photons can lead to the death or inability to reproduce of organisms. This effect is particularly significant for killing bacteria, insects and other harmful organisms that can be present in or on products.
Common applications for gamma radiation treatment include the sterilization (killing bacteria) of medical products, reduction in the microbial count of foods, cosmetics and their packaging, and pest control in agricultural products.
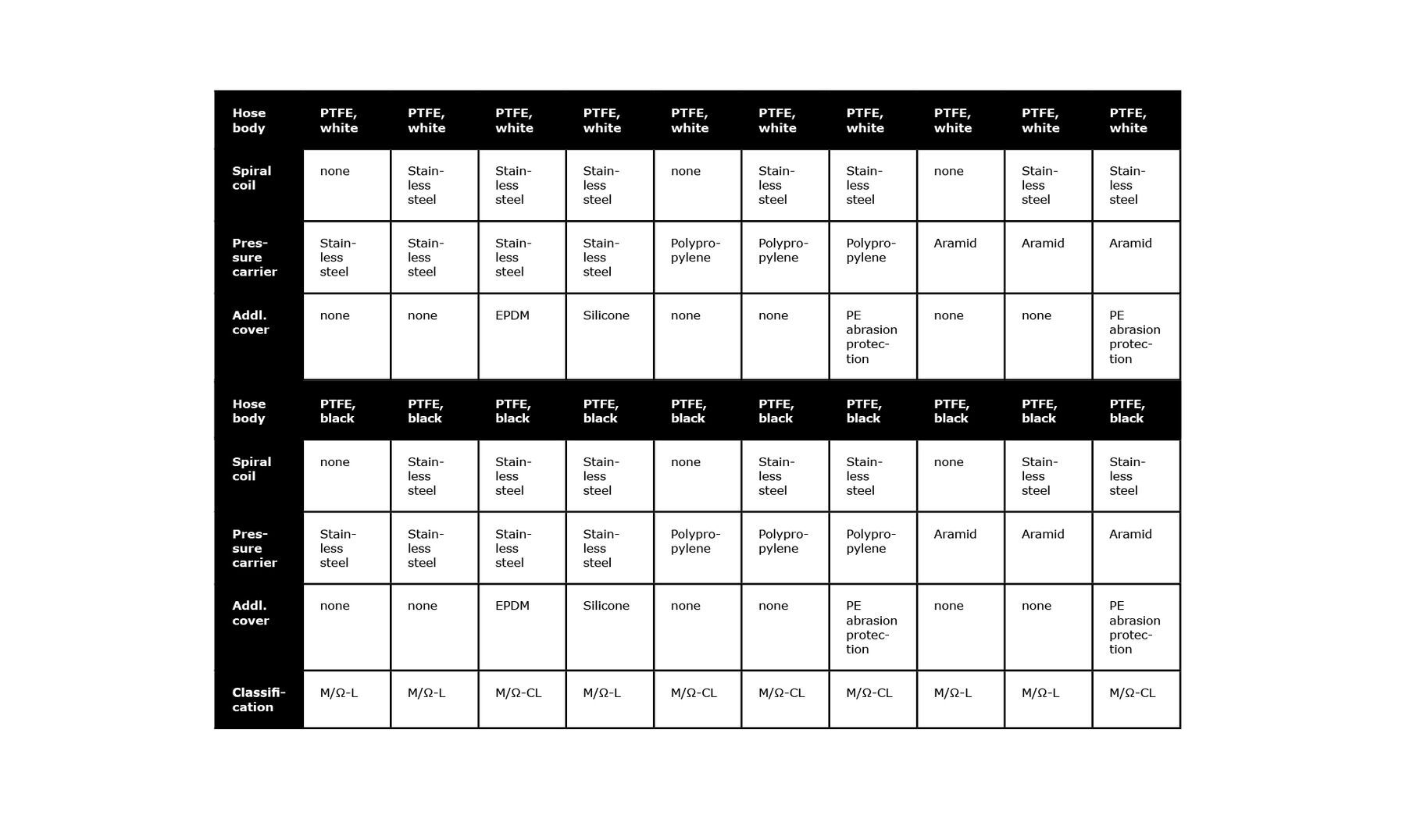
10. Nominal diameters and conductivity
The hose must be selected with the right rated diameter for the anticipated volume fl ow. Markert offers a wide range of hose diameters.
- DN 19 (3/4“)
- DN 25 (1“)
- DN 32 ( 11/4“)
- DN 38 (11/2“)
- DN 50 (2“)
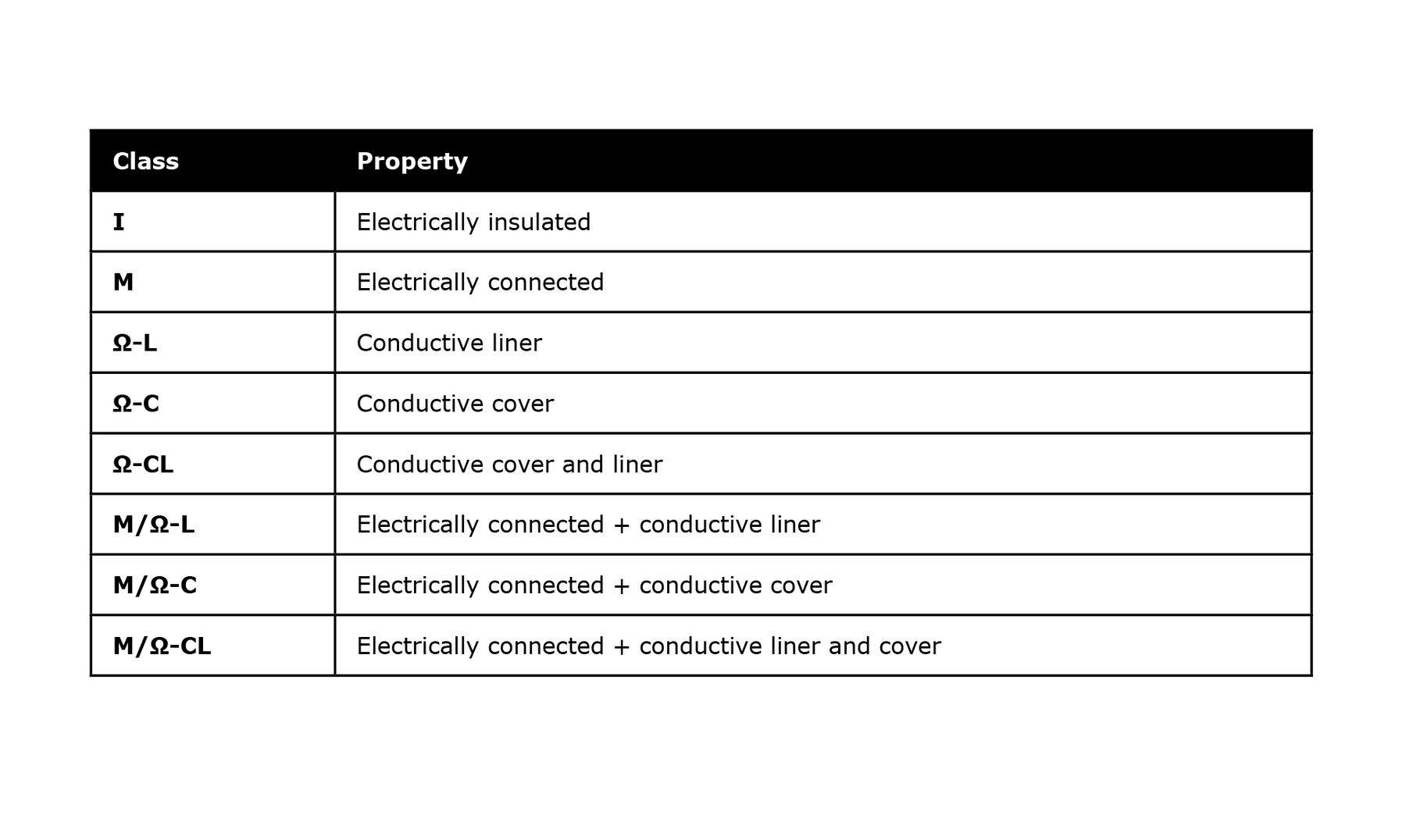
PTFE hoses can build up static electric charges. This makes the conductivity of the hose important. To make the PTFE liner conductive, carbon can be mixed into the PTFE material during manufacture. This gives a conductive, black PTFE liner. In addition to the conductivity of the liner, the conductivity of the covering structure can also be important. The following table provides an overview of various liner/cover variants and their respective conductivity classes.